Ozempic drug to treat diabetes.
Courtesy: Ozempic
In their most recent earnings reports, Novo Nordisk and Eli Lilly proved why they are the undisputed heavyweights in the prizefight for control of the rapidly growing weight-loss medications market. Beyond staggering sales figures for Novo’s Ozempic and Wegovy and Lilly’s Mounjaro, news of a study showing Wegovy can reduce the risk of heart disease and the anticipated approval of even more powerful prescription drugs to treat obesity will only strengthen the position of these venerable pharmaceutical giants, which have been in business for 100 years and 147 years, respectively.
Even so, their competitors are not ceding the market to the current leaders. Pfizer, Amgen and other pharmaceutical companies are rushing to develop weight-loss drugs, though they may not be available for another year or more.
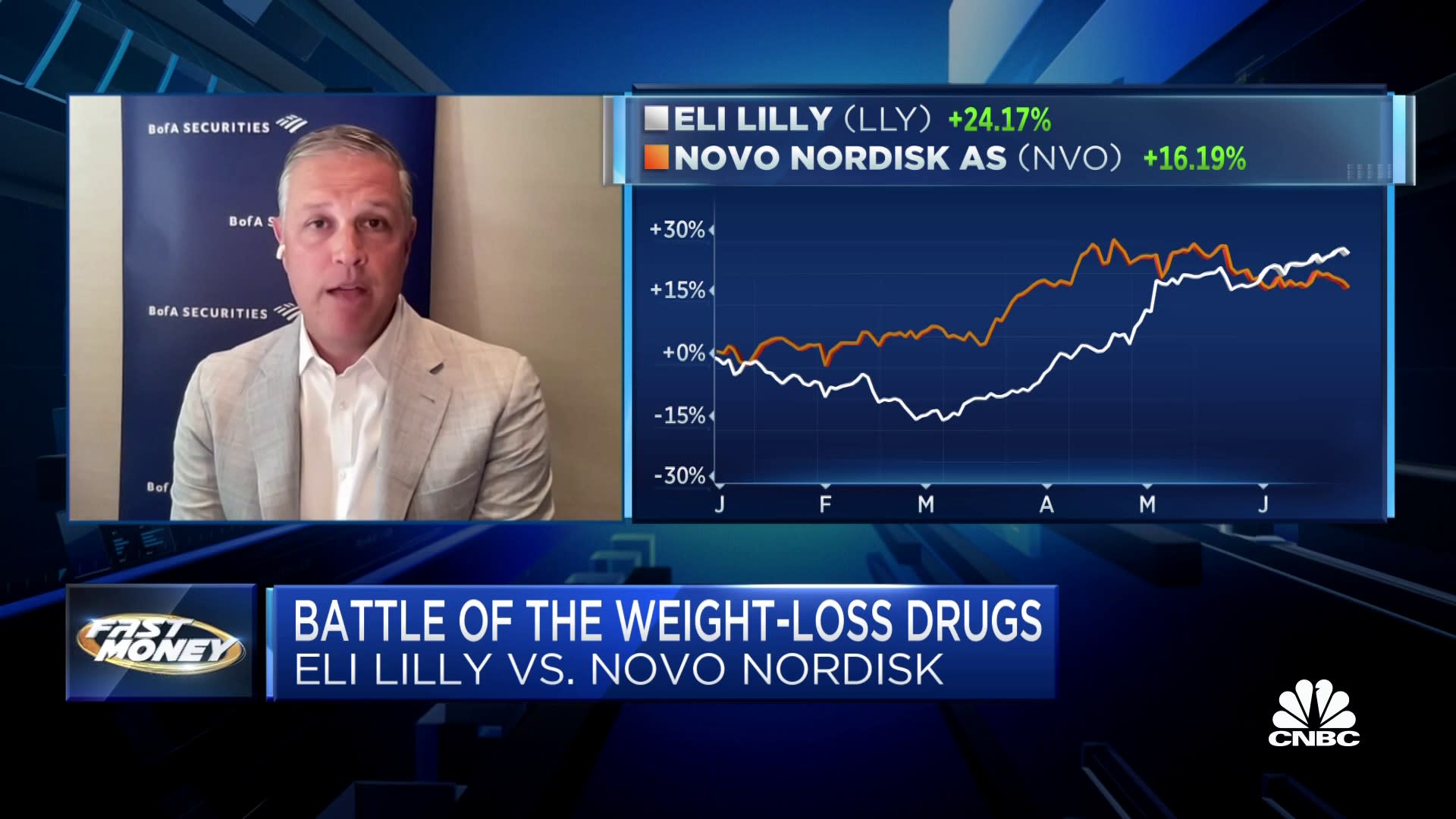
“We think it’s Novo and Lilly, and everyone else is just scrambling to keep up,” said Emily Field, head of European pharmaceutical equity research at Barclays, which predicts a $100 billion global market for this class of drugs by around 2030. Since Wegovy and Mounjaro have been on the market, “neither company can make the drug fast enough,” she said. “For both, demand has outpaced even their most bullish assumptions of what they’re able to supply.”
“It’s my top priority … expanding the capacity of our ability to make not just Mounjaro but other drugs like it in our pipeline to meet the challenge here,” Lilly CEO David Ricks told CNBC’s Jim Cramer in August.
Ozempic and Wegovy are both GLP-1 agonists, substances originally formulated by Novo to treat type 2 diabetes. Besides controlling blood sugar, GLP-1s affect hunger signals to the brain, tricking the body into feeling full and slowing the rate at which the stomach empties. The sales growth has been so sharp that Novo Nordisk contributed the majority of recent economic growth for its home nation of Denmark.
The Food and Drug Administration approved Ozempic in 2017 for diabetes and Wegovy in 2021 to treat obesity. Over time, both reduce body weight by about 15%. Mounjaro, introduced in 2022 to treat diabetes, contains GLP-1, plus GIP, a similar appetite suppressor that can lead to weight loss. All three drugs are prescribed as injectable pens that patients self-administer weekly.
On August 8, Lilly reported that its second-quarter income jumped 85% from the same period a year ago, driven in large part by Mounjaro, which generated $979.7 million in sales for the quarter, compared to $16 million in the year-ago period and $569 million in the first quarter of this year. In December, analysts at SVB Securities projected that Mounjaro sales could reach $26.4 billion by 2030.
A month earlier, Lilly released data from a phase three trial of the drug, showing that it helped patients with obesity, though not diabetes, lose up to 26.6% of their body weight after 84 weeks of treatment. Mounjaro is currently only approved by the Food and Drug Administration to treat diabetes, but the company has filed for FDA approval of Mounjaro specifically to treat obesity, which could come later this year or in early 2024.
A pharmacist displays a box of Mounjaro, a tirzepatide injection drug used for treating type 2 diabetes made by Lilly at Rock Canyon Pharmacy in Provo, Utah, March 29, 2023.
George Frey | Reuters
Novo traded earnings jabs with its opponent on August 10, reporting that in the first six months of this year, sales of Wegovy soared 344% in the U.S. alone to nearly $1.7 billion, while sales of Ozempic jumped 50% to more than $3.7 billion. According to financial analyst firm FactSet, sales of Wegovy and Novo’s other weight-loss injectable, Saxenda, could reach $6.1 billion before the year is out and $15 billion annually by 2030. (Saxenda, on the market since 2015, reduces weight by 6%-8%.)
Potentially bigger news came a couple of days earlier, when Novo released headline results of SELECT, a multi-year clinical trial of Wegovy, showing that it reduced the risk of major cardiovascular events such as heart attacks or strokes by 20%, compared with a placebo. During the earnings call with analysts, Novo CEO Lars Fruergaard Jorgensen said that the SELECT trial “underlines the importance of recognizing obesity as a serious chronic disease.”
The company expects to file for regulatory approvals of a label indication expansion for Wegovy in the U.S. and the European Union this year, adding the drug’s cardiovascular benefits.
The search for new potential uses of these drugs with difficult health care issues continues, with the latest headlines saying obesity drugs are now being investigated as potential treatments for dementia and addiction, too.
Heart benefits, and doctor pressure, may force Medicare to cover
Results like these may well prod government and private insurers to reimburse more patients for the high price tag of weight-loss prescriptions. By law, Medicare hasn’t covered them since 2006, though some select Medigap and Medicare Advantage plans for retirees do. As of late July, Medicaid programs in nine states covered Wegovy, which costs $1,349 monthly. Private insurers often do not cover GLP-1 drugs prescribed for weight loss only.
Conversely, Medicare, Medicaid and most private insurers cover Ozempic ($936 per month) when it’s prescribed for type 2 diabetes treatment, but not for weight loss. Coverage for Mounjaro ($1,023 per month) to treat diabetes varies based on an individual’s insurance plan and drug benefits.
Based on the SELECT study — Novo will release full results later this year — most payers may ultimately cover weight-loss drugs if a patient’s provider prescribes them to treat or prevent cardiovascular conditions, which can be exacerbated by diabetes and obesity.
“They’re going to have no choice at this point,” said Dr. Wajahat Mehal, director of the Yale School of Medicine’s Metabolic Health and Weight Loss Program, whose patients respond well to GLP-1s. The SELECT results “are going to put pressure on insurance companies” to cover those drugs, he said.
On July 20, a bipartisan group of U.S. senators and representatives reintroduced the Treat and Reduce Obesity Act, which would reverse the federal ban on Medicare coverage of obesity drugs. “With obesity rates on the rise in our country, we must do more to combat this epidemic head on,” said Democratic Senator Tom Carper of Delaware. “Too many of those in need are being denied care because of the high cost of medications or inaccessible treatment options.” Added Republican Senator Bill Cassidy of Louisiana, “Expanding Medicare coverage to the treatments patients need enables them to improve their health and benefits us all.”
Novo and Lilly have lobbied Congress on behalf of the legislation, originally introduced in 2021, and have endorsed its reintroduction. Novo has also courted members of the Congressional Black Caucus and influential Black Americans to have Medicare cover weight-loss drugs. According to the CDC, nearly 50% of non-Hispanic Black adults are classified as obese, the highest rate for any race or ethnicity.
The push by pharma giants for expanded coverage of these new blockbusters comes as the federal government is for the first time forcing drug companies to negotiate with Medicare over some of their most successful products, and several recent news reports speculated that Ozempic will be a top target for negotiated prices in the future. The pharmaceutical industry is challenging the new law’s constitutionality in court.
Attitudes about obesity being transformed
Overall, per the CDC, obesity in the U.S. affects 100 million (41.9%) adults and 14.7 million (19.7%) children and accounts for approximately $147 billion in annual health care costs. Historically, obesity has been considered a behavioral and lifestyle condition among people lacking the willpower to moderate eating habits and exercise regularly. But those attitudes have been changing, not only in society at large but also among health care providers.
In 2013, the American Medical Association recognized obesity as a disease, which was a huge step forward in the field of medicine, as well as for pharmaceutical companies to develop a new generation of weight-loss drugs that are more effective and safer than other obesity products, such as Contrave, Orlistat, Qsymia and Imcivree.
“We’ve actually been in obesity research for more than 25 years,” said Camilla Sylvest, executive vice president, commercial strategy and corporate affairs at Novo. “A lot of things have changed in society in the meantime, not only that we have new weight-loss products on the market, but there’s also a much greater understanding of why obesity is a disease and why it needs to be treated.”
All that has increased the interest in treating obesity, “and therefore demand for Wegovy has exploded,” Sylvest said. Indeed, new prescriptions for Wegovy and Ozempic have risen by 297% percent and 140%, respectively, since a little more than a year ago, according to analysts at Cowen. What’s more, Novo and Lilly have each seen their stock prices more than double in the past three years.
It’s no surprise, then, that Novo and Lilly have had trouble keeping up with demand for their weight-loss drugs. In May, Novo reduced the U.S. supply of Wegovy starter doses to ensure access for existing patients, and in its recent earnings report said it expects that to continue into the fall. Likewise, in its second-quarter earnings call, Lilly executive vice president Mike Mason told analysts, “We do still expect to see tight supply and some spot outages on Mounjaro through the end of the year.”
In 2021, Novo announced plans to invest nearly $2.6 billion to build three new ingredients manufacturing facilities and to expand an existing production site in Kalundborg, Denmark. Then last May, the company committed $2.3 billion to expand its site in Hillerød, Denmark. In April, Lilly said it is investing an additional $1.6 billion in its two new factories in Boone County, Indiana, bringing the company’s total commitment to the site to $3.7 billion.
Meanwhile, neither Big Pharma giant is resting on its weighty laurels, as both have increased their R&D budgets toward oral versions of their diabetes and obesity drugs, targeting patients who would prefer a pill instead of a jab. Plus, pills taken once daily may be cheaper to manufacture than single-dose pens used weekly.
Novo is in phase three clinical trials of oral versions of GLP-1, which it says could be even more effective in treating type 2 diabetes and obesity. It expects to file for FDA approval later this year.
At an American Diabetes Association event in June, Lilly released phase two data for orforglipron, its first oral drug for obesity, saying it achieved up to 14.7% weight reduction after 36 weeks. The company also released phase two data from trials on retatrutide, an injectable obesity medication that achieved up to 17.5% weight reduction after 24 weeks.
Pfizer was testing two different oral drugs to treat type 2 diabetes and obesity, but is now focusing on one, danuglipron, after phase two trial results. “While we have our data from the type 2 diabetes study [of danuglipron], we are still studying the potential in obesity,” a Pfizer spokesperson said in an email. “When we have this information, which should be later this fall, we will make a plan for what the phase three [trial] will look like.”
In December, Amgen reported that in phase one tests of its injectable obesity drug, AMG133, patients showed a weight loss of 14.5% after 12 weeks of treatment. Phase two trials are ongoing, with data expected in 2024, and a product launch is not likely before 2026.
So as their competitors continue to develop competitive therapies for diabetes and obesity, “Novo and Lilly will duke it out,” said Emmanuel Papadakis, an industry analyst at Deutsche Bank.
Once they can supply enough products to keep up with the overwhelming demand, don’t be surprised to see direct-to-consumer ads for Wegovy and Mounjaro popping up on the evening news. “Both companies over time will be investing considerably to establish their marketing presence,” he said, “to the extent that it’s needed.”
This article was originally published on CNBC

