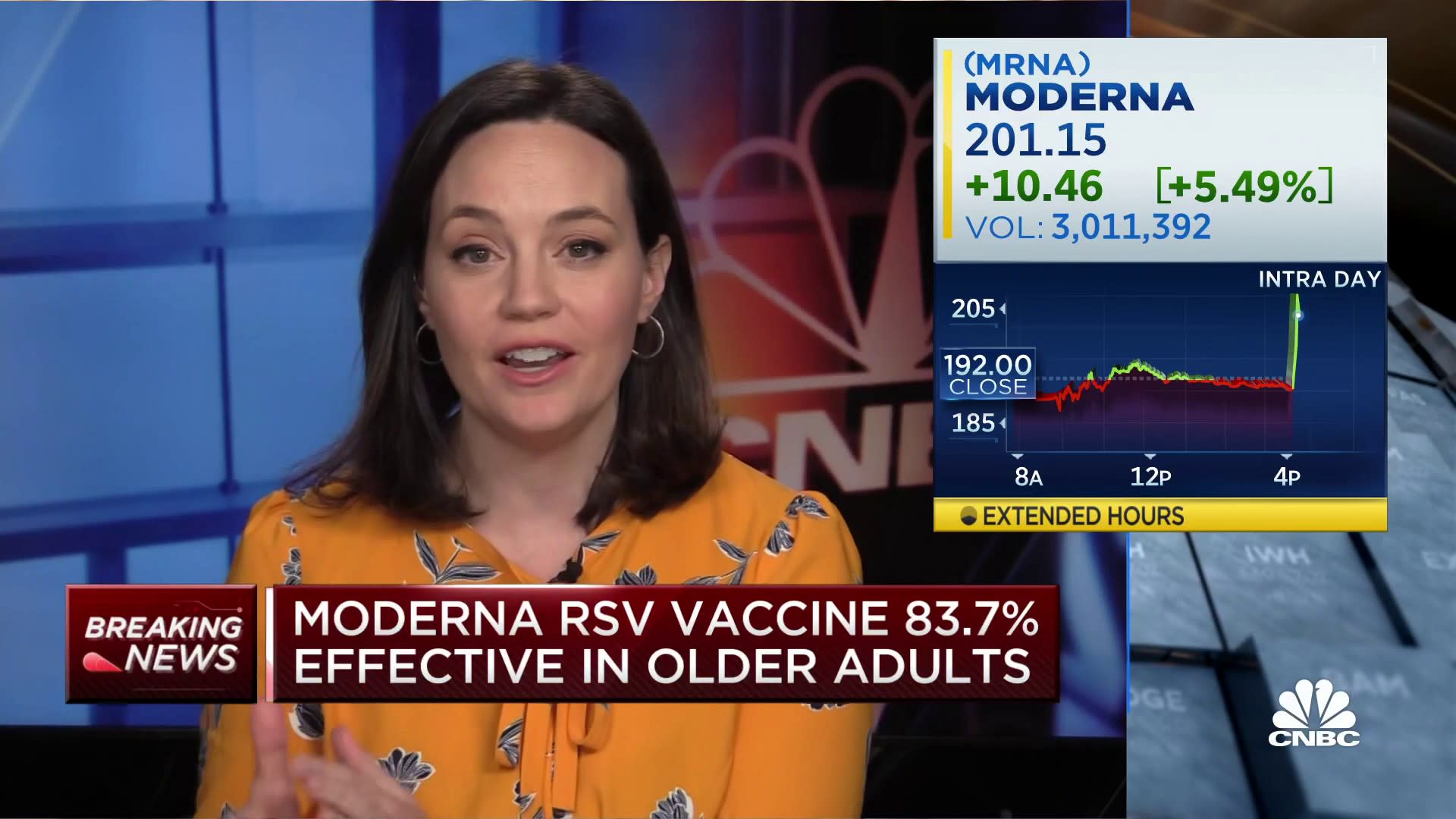
Moderna on Tuesday said its vaccine that targets respiratory syncytial virus is effective at preventing disease in older adults.
The vaccine was 83.7% effective at preventing lower respiratory tract disease, defined as two or more symptoms, in people ages 60 and older, according to the Boston biotech company. It was 82.4% effective at preventing lower respiratory tract disease with three or more symptoms.
No safety concerns have been identified during the clinical trial of the vaccine, according to Moderna. The safety and efficacy data from the trial will be published in a peer-reviewed journal, according to the company. The clinical trial has enrolled about 37,000 people across 22 countries.
Moderna said it plans to file an application for approval by the Food and Drug Administration in the first half of this year. There currently is no FDA-approved vaccine for RSV.
Moderna’s stock rose nearly 7% in extended trading.
RSV infections kill between 6,000 and 10,000 older adults every year and result in 60,000 to 120,000 hospitalizations, according to the Centers for Disease Control and Prevention.
The U.S. suffered an unusually severe RSV season in the fall among children and older adults as the public largely stopped practicing public health measures implemented in response to the Covid-19 pandemic, such as masking and social distancing.
Moderna’s RSV vaccine uses the same messenger RNA technology as the company’s successful Covid shots. The Covid vaccine turned Moderna into a global name and delivered windfall profits, but it remains the company’s only commercially available product and demand is fading.
The Boston biotech company faces growing pressure to demonstrate that other products in its pipeline will successfully come to market. Morgan Stanley estimates the market for an adult RSV vaccine is $7 billion to $10 billion.
This article was originally published on CNBC

